Macular Degeneration
What is macular degeneration?
The macula is the central area of the retina where reading vision is located. The retina is the light sensitive inner lining in the back of the eye that is like the film in a camera. Anatomically, the macula is located in the straight-ahead position of the eye and is at the center of the retina. If the retina were a target, the macula would be the bull’s eye.
Macular degeneration causes a person’s vision to become blurred, distorted, and/or dark in the straight-ahead direction. While macular degeneration rarely causes loss of all vision, it can make daily activities such as reading, driving, and recognizing faces difficult or impossible.
What causes age-related macular degeneration?
Macular degeneration is almost always seen in older people, although in a few families it may start earlier. The prevalence of macular degeneration increases as the population gets older. There is a definite genetic predisposition as shown by studies comparing identical twins and fraternal twins. Other factors that have an effect on the severity of the disease include high-blood pressure and cigarette smoking. It is now the most common cause of legal blindness in retirement- age people in the United States. There are two main forms of macular degeneration: dry and wet.
What is the difference between wet and dry macular degeneration?
Most people who suffer from macular degeneration have the “dry” form. Dry macular degeneration involves changes in the pigment layer of the retina in the back of the eye and the accumulation of yellow deposits called drusen. More light may be required for reading, and it may take longer to adapt to a darker room. Most people maintain the ability to read and drive, but occasionally dry macular degeneration can cause legal blindness when it reaches the advanced dry age-related macular degeneration stage known as geographic atrophy.
Geographic atrophy (Advanced dry AMD) is a progressive, irreversible loss of the retinal cells in the macula, which are responsible for central vision. Approximately 20% of individuals with macular degeneration have the advanced dry macular degeneration form of geographic atrophy.
Wet macular degeneration accounts for about 10-15% of all cases. Wet macular degeneration is the more severe form of the disease. People with wet macular degeneration develop abnormal leaking blood vessels and membranes under the macula. If uncontrolled, the bleeding may result in scar formation and irreversible vision loss.
With wet macular degeneration, vision loss is often rapid and severe. The wet form can be further classified into classic, occult, polypoid, and Retinal Angiomatous Proliferation (RAP).
What are the symptoms of wet macular degeneration?
Dry macular degeneration is usually a slow process and is hardly noticeable in its early stages. It is important to detect the transition from dry to wet macular degeneration as soon as possible to get the best effect from vision-saving treatments. While symptoms may vary from person to person, there are several ways to detect macular degeneration. These methods work for both wet and dry macular degeneration but a recent onset or worsening of symptoms suggests wet macular degeneration.
Common symptoms include: 1) dark areas or holes in the center of your vision, 2) letters or words begin to look blurry when reading and 3) straight lines appear distorted or wavy. One simple way to check for macular degeneration is to use the Amsler grid.
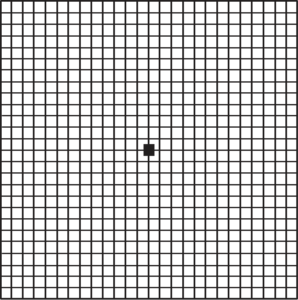
Amsler Grid
Instructions for using the Amsler Grid to test your vision:
- Under a good light source, put on your reading glasses (if any) and hold the grid about 1 foot from your
- Cover one With the other eye, look directly at the center dot on the grid.
- Note if any of the straight lines ap- pear wavy or distorted, or if there are dark or blurred sections of the
- Repeat the procedure with your other
- If you notice blurry, dark or distorted sections on the grid, contact your eye doctor
How is wet macular degeneration diagnosed?
Because the early symptoms of wet macular degeneration usually occur in one eye at a time, the good vision in the other eye may mask the symptoms of the eye with wet macular degeneration. Many people don’t realize they have the disease until their visual loss is already severe. However, by using the Amsler grid frequently to test your eyes and by testing each eye separately, you can spot the disease early in its course. Regular eye exams also detect early signs of the disease.
If you or your physician suspect wet age-related macular degeneration, your eye doctor can use a microscope and ophthalmoscope to get a detailed view of your macula. Another method to detect abnormal blood vessels in the retina is a special series of eye photographs, called a fluorescein angiogram (Fig. 1), which are taken as a dye is injected in a vein. Indocyanine green angiography is a similar test which better demonstrates occult and polypoidal macular degeneration and persistent larger choroidal feeder vessels. Optical coherence tomography (Fig. 2) is a laser scan that demonstrates fluid accumulation in wet macular degeneration.
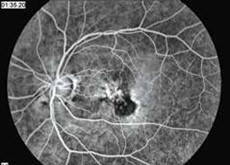
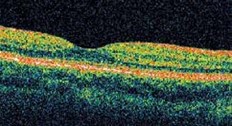
How is wet macular degeneration usually treated?
Anti-Angiogenic Drug Injection
For patients with all forms of wet macular degeneration under the center of vision, anti-angiogenic drug injection is usually the therapy of choice. The FDA has approved Lucentis, Eylea and Vabysmo which are Vascular Endothelial Growth Factor (VEGF) inhibitors, for injection into the eye. A fourth VEGF inhibitor, Avastin, has been approved by the FDA for injection into veins as a cancer therapy, and is used as an “off-label” therapy for wet macular degeneration as well. However, it is formulated in a compounding pharmacy, which may have additional risks as compared to medications prepared specifically for eye injections by pharmaceutical companies. A relatively painless technique to inject a small amount of the VEGF inhibitor into the vitreous cavity of the eye has been developed.
Lucentis Injection
Lucentis is specifically approved for injection into the eye and has a high level of proof. In many FDA approved studies; 95% of patients maintained vision, 75% had some improvement in vision and 40% had a large improvement in vision, where they were able to maintain vision to legally drive.
Lucentis injections are given every 4 weeks. When Lucentis injections were stopped after only 3 injections, 80% of patients required retreatment because the vision, which had initially improved, worsened again. Even after 3 years of monthly treatments, some patients lost vision when the treatment was not continued. The best visual results are achieved with monthly injections over the first year of treatment. After one year, it may be possible to extend the treatment interval, but this varies from patient to patient.
Retina Associates of Florida completed a review of patients receiving continuous fixed interval dosing treatment with anti-VEGF medications for at least 15 years (FIDO trial), and visual acuity improvements were maintained in the vast majority of patients that received continuous, uninterrupted therapy.
Eylea Injection
Eylea is another VEGF inhibitor approved for intraocular injection in late 2011. The package insert states that it can be used every month or every 2 months. This suggests that Eylea has a longer duration than Lucentis or Avastin, but studies show that Lucentis and Eylea have the same duration of effect.
Vabysmo Injection
Vabysmo (faricimab, Genentech) is a more recent medication FDA approved for the treatment of wet age-related macular degeneration. Vabysmo is an antibody that acts against both VEGF and Angiopoietin-2 (Ang-2). Some patients may require less frequent injections with Vabysmo. Almost 80% of patients in the clinical trial were able to be extended up to a 3-month interval at 2 years of treatment. Retina Associates of Florida was involved in this clinical trial and is proud to have been able to provide this cutting edge treatment earlier to patients.
Avastin Injection
Prior to the release of Lucentis by the FDA, some doctors tried injecting an anti-cancer drug with anti-VEGF properties into the eye and found that it was helpful for patients with macular degeneration. A study (CATT) was conducted to test its effectiveness. After
2 years of treatment, Avastin given less than monthly was found to be inferior to Lucentis monthly. Because Avastin is compounded by local pharmacies, there has been a higher risk of loss of vision from infections caused by Avastin that became contaminated during the compounding process. Also, because Avastin does not meet the FDA sterility guidelines for intraocular injection, there is potential for inflammation or toxicity from its use. Since Avastin is approved by the FDA for cancer use in humans, the law allows doctors to use it as they think appropriate. In order to use it in the eye, we need to inform the patient that it is “off-label” and of all the risks associated with “off-label” use. An increased risk of strokes and heart attacks has been noted when it is used intravenously to treat cancer. Avastin is much cheaper and Medicare usually pays for its use in macular degeneration, but since it is “off-label” they are not obligated to pay. We use Avastin for macular degeneration most often in patients who would have large out-of-pocket expenses for Lucentis, Eylea or Vabysmo. Ultimately it is up to the patient to choose which drug they would like to use. However, it is becoming increasingly common for insurance companies to decide which drug the patient receives.
Biosimilar Therapy
Biosimilar medications are biologics that are very similar to the original medications produced by separate companies. The patent for Lucentis and Eylea has expired and there are currently biosimilar treatments for both medications. Similar to Avastin, insurance companies may decide which drug the patient receives. Our front desk will work with your insurance company to figure out which medication is allowed for your condition.
Surgical Device Implantation
Susvimo (ranibizumab, Genentech) is a port delivery system that is surgically implanted and is recently FDA approved for the treatment of wet age-related macular degeneration. The implant allows sustained release of ranibizumab. Patients typically receive an in-office refill of the surgical implant every 6 months. This surgical procedure helps limit the number of injections patients need and may be a suitable option for some patients. Retina Associates of Florida was one of the leading recruiting sites for this innovative treatment.
Are there any new treatments on the horizon for wet AMD? YES!
1.) We are currently involved in a Phase II trial (Eyp-1901) for an injection in the eye that provides sustained delivery of anti-vascular endothelial growth factor (anti-VEGF). This trial is promising as it could potentially limit the number of injections patients receive to just one injection every 6 months.
2.) Macular surgery to implant new pigment cells or stem cells under the center of vision have shown variable results, but are in a very early stage of development.
What if I have a macular hemorrhage?
For small hemorrhages, standard anti- VEGF therapy is usually sufficient. For larger hemorrhages, a gas bubble and/ or TPA (Tissue Plasminogen Activator) can be injected into the eye or under the macula with a small glass bubble to liquefy and massage blood clots out from under the macula.
What about aspirin and other anticoagulant medicine?
Taking aspirin and other blood thinning medications is not thought to cause bleeding in wet macular degeneration; however, it probably makes the amount of hemorrhage larger when it occurs. There are no restrictions on blood thinners in dry macular degeneration.
In patients with wet macular degeneration, the risks and benefits of reducing blood thinning medications should be explored. If there is a good indication for the medication, it should be continued.
Are there any treatments available for dry macular degeneration?
A study by the national Eye Institute showed that a daily dose of antioxidant vitamins C 500 mg, and E 400 IU plus zinc oxide 25 mg and cupric oxide 2 mg, 10 mg lutein and 2 mg zeaxanthin, reduced the risk of dry macular degen- eration progressing to wet macular de- generation by 25%. Therefore, taking these supplements, which are called “AREDS2”, is recommended once many or large drusen develop or if wet macular degeneration has already developed in one eye. Commercial prepara- tions with the correct formula include: Ocuvite, Preservision and I-Caps ARED2 formula. Although taking a low dose multi vitamin with AREDS2 formula is acceptable, do not take extra doses of Zinc or Vitamin C and E. If a patient has wet AMD in both eyes, the benefit of AREDS2 is less known. Smokers should strive for cessation and consider a formulation of vitamins without A, as it has been associated with a higher risk of lung cancer.
Syfovre (Pegcetacoplan) injection
Syfovre (Apellis) is a complement 3 inhibitor that is injected into the eye and was shown to reduce the rate of growth of geographic atrophy by around 24% at 2 years. Geographic atrophy is the advanced stage of dry age-related macular degeneration. This is the first FDA approved treatment that can slow the rate of progression of atrophy. Make sure to discuss with your physician if you are a potential candidate to receive this treatment. Retina Associates of Florida was involved in this clinical trial and is proud to have been able to provide this cutting edge treatment earlier to patients.
Izervay ( avacincaptad pegol) injection
Izervay is a complement 5 inhibitor that is injected into the eye and was also shown to reduce the rate of growth of geographic atrophy by around 18-28% at year 1. Izervay and Syfovre have overall similar outcomes and side effect profiles from their clincal trial results and are both exciting novel treatments for patients with the advanced form of dry AMD (geographic atrophy).
What if vision is reduced despite the best treatment?
Low vision devices can often greatly improve visual performance in people that are considered legally blind. The two major classes of low vision devices are 1) lens magnification combined with a brighter light and 2) Closed Circuit Television (CCTV ) with electronic magnification and image intensification. Reputable providers of low vision devices usually present a selection of options, not just a single, very expensive low vision device. Reputable providers also will allow the devices to be returned if they are not found to be helpful.
Implant telescopic low vision devices have been approved by the FDA. Patients have to be legally blind in both eyes to qualify.
